Supplements containing synthetic ingredients or excipients have been around for a long time. Most people in the industry define synthetic supplements as supplements made from ingredients that were created in a lab. Others define synthetic supplements as any supplements that aren’t whole food supplements. Which is right? Arguably, they both are, but when thinking about the labeling quandary, it’s mostly related to supplements made from synthetic ingredients – i.e., those that either you won’t find them anywhere in nature or are petrochemical-based versions of those in nature.
Synthetic supplements aren’t inherently bad—in some cases, you can only get the supplement if it’s made synthetically. In fact, some ingredients may be more sustainable, or eco-friendly when produced through non-natural processes. Industry Transparency Center, as its name implies, believes that ingredients should be labeled synthetic as a standard and best business practice.
In 2018, ITC surveyed 1,002 consumers to ask them if they thought synthetic ingredients and supplements should be labeled. The answer at that time was overwhelmingly yes – 83 percent of respondents said synthetic supplements should always be labeled.
We recently reissued the survey—this time to consumers in both the US and the UK and the results were markedly different. As the chart below shows, among all US and UK respondents, 62% said to always label synthetic supplements, down from 21% from the 83% who responded that way in 2018.
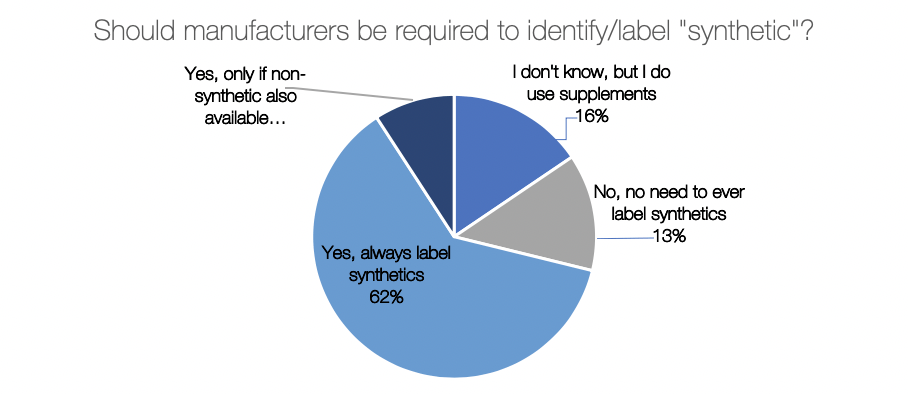
There were some interesting differences when we compared responses across gender in the US and UK. US women said to always label synthetic much more than US men, or expressed that they weren’t sure. Nearly 20% of UK men felt there was no need to ever label synthetics.
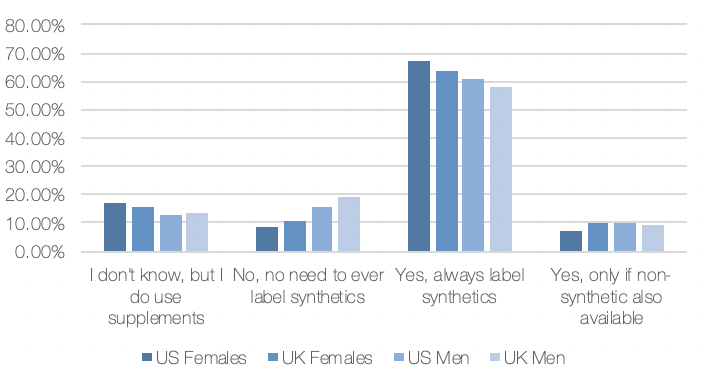
Breaking it down by age, those aged 55 to 64 were the most opinionated – over 70% felt that synthetic supplements should always be identified and fewer than 50% of those in the 25 to 34 year old age group were concerned about labeling.
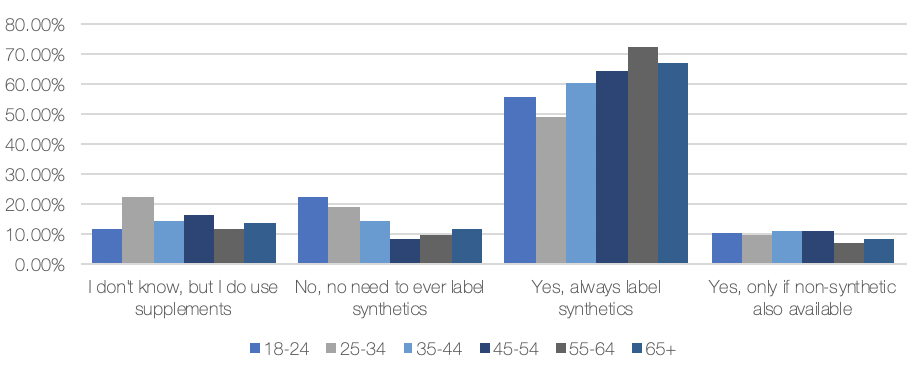
So why the big change from 2018?
Well, obviously there are quite a few consuming issues occurring globally right now. There are also media articles like this Healthline article that make a case that nearly all supplements are synthetic so if this is truly reflective of consumers being under that mindset, it won’t matter.
I also turned to ITC Founder Scott Steinford and CEO Len Monheit for their thoughts:
Len shared:
The overall result, while a little surprising, I’d explain by saying that there are driving concerns that have lowered prioritization of transparency regarding synthetic ingredients and supplements. Other surveys have described availability, reliability and trust as increasing as consumers make brand decisions. I think too that this industry has so much more education to do about where products come from and how they are actually produced, and we need to get much more ahead of that for when priorities change again. This is going to be more important as we try to retain the new consumers who are now exploring our category. They’ll become even more discerning, which is good, and we have to be ready for that conversation. In the meantime, we need to keep pushing transparency as a trust-building tool.
Scott shared:
In most cases the distinction between natural and synthetic is easily discerned. If an ingredient molecule is identical to what is produced in nature, the ingredient can more easily be considered “natural”. If, on the other hand, the ingredient molecule is, in any way different, the question of natural versus synthetic becomes more subjective. While our most recent survey did not indicate the same level of desire to have the synthetic distinction called out, in both surveys, and others like it, a majority seeking identification of synthetic prevails. ITC has not fielded a question of “How important is it to you, on a scale of 1-10, to know if the ingredient you are buying is synthetic or natural?” We have had many conversations surrounding this question over the years and it is clear, to me at least, the people who do have an opinion of identification, are much more adamant on the subject than those who do not. Also, it appears the way the question is answered is more often a result of how informed the responder is to the subject. The more research conducted by the responder into the topic results in a greater propensity to answer a higher desire to provide more complete transparency. In general, consumers want more transparency than less. If there is a clear distinction between natural and synthetic, such as with vitamin E, there should be, and is, a requirement to identify the difference. Most other ingredients, even if it is a clear determination between synthetic and natural, do not have this requirement. We are not without the precedent of the requirement to identify synthetic, but we are still short of the definition of synthetic. As an industry, we should work to better define synthetic versus natural.
Once the pandemic ends and we return to a more standard priority environment (or not), we’ll reissue this survey and see if results change.
Readers, what are your thoughts?
~Traci

